L/N/K-Selectride

The reagents having the formula M[HB(sec-Bu)3] are used as reducing agents. There are three types with different cations: L-Selectride (lithium), N-Selectride (sodium), and K-Selectride (potassium).
Because of the sec-butyl groups, these reducing agents are sensitive to steric influences and often allow for chemo- and stereoselective reductions.
The chemoselectivity of M-Selectride reduction is strongly influenced by the steric and solvent factors.

The stereoselectivity is particularly high in the reduction of conformationally restricted cyclic ketones.

The stereoselectivity follows the Felkin-Anh model.

Diisobutylaluminium hydride
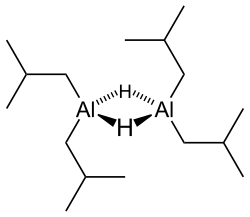
DIBAL is useful in organic synthesis for a variety of reductions, including converting carboxylic acids, their derivatives, and nitriles to aldehydes. DIBAL efficiently reduces α-β unsaturated esters to the corresponding allylic alcohol. By contrast, LiAlH4 reduces esters and acyl chlorides to primary alcohols, and nitriles to primary amines [using Fieser work-up procedure]. DIBAL reacts slowly with electron-poor compounds, and more quickly with electron-rich compounds. Thus, it is an electrophilic reducing agent whereas LiAlH4 can be thought of as a nucleophilic reducing agent.
Although DIBAL reliably reduces nitriles to aldehydes, the reduction of esters to aldehydes is an infamously finicky reaction which looks useful on paper but often leads to mixtures of alcohol and aldehyde in practice. Nevertheless, it is possible to avoid these failures through careful control of the reaction conditions using continuous flow chemistry.
Sodium bis(2-methoxyethoxy)aluminium hydride
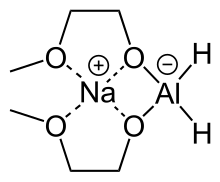
Sodium bis(2-methoxyethoxy)aluminium hydride (SMEAH; trade names Red-Al, Synhydrid, Vitride) is a complex hydride reductant with the formula NaAlH2(OCH2CH2OCH3)2. The trade name Red-Al refers to its being a reducing aluminium compound. It is used predominantly as a reducing agent in organic synthesis. The compound features a tetrahedral aluminium center attached to two hydride and two alkoxide groups, the latter derived from 2-methoxyethanol. Commercial solutions are colorless/pale yellow and viscous. At low temperatures (<-60°C), the solution solidifies to a glassy pulverizable substance with no sharp melting point.
SMEAH is a versatile hydride reducing agent. It readily converts epoxides, aldehydes, ketones, carboxylic acids, esters, acyl halides, and anhydrides to the corresponding alcohols. Nitrogen derivates such as amides, nitriles, imines, and most other organonitrogen compounds are reduced to the corresponding amines.

As a reagent, SMEAH is comparable with lithium aluminium hydride (LAH, LiAlH4).
It is a safer alternative to LAH and related hydrides. SMEAH exhibits similar reducing effects, but does not have the inconvenient pyrophoric nature, short shelf-life, or limited solubility of LAH. Upon contact with air and moisture, SMEAH reacts exothermically but does not ignite, and tolerates temperatures up to 200 °C. Under dry conditions it has unlimited shelf life. It is soluble in aromatic solvents, whereas LAH is only soluble in ethers. For example, a solution greater than 70 wt.% concentration in toluene is commercially available. The reagent can be modified to effect partial reductions.
SMEAH in toluene under reflux has been used to reduce aliphatic p-toluenesulfonamides (TsNR2) to the corresponding free amines and is one of the few reagents that can carry out this challenging reduction in general settings. Notably, LiAlH4 does not reduce this functional group unless forcing conditions are used.




