 |
| Remdesivir packing in Chinese |
| Bin Cao and ISARIC |
 |
| Zhengli Shi in WIV P4 Lab |
 |
| Lopinavir/ritonavir's Price in China |
| Clinical trial protocol Peter Horby involved | ||||
| Protocol | Dosage/12 hours | Course | Total number of patient | Conclusions |
| China | Lopinavir 400mg - Ritonavir 100 mg | 10 Days | 199 | In hospitalized adult patients with severe Covid-19, no benefit was observed with lopinavir–ritonavir treatment beyond standard care. |
| UK | Lopinavir 400mg - Ritonavir 100 mg | 10 Days | 11500 | No result published by now. |
 |
| Hydroxychloroquine,Chloroquine |
 at the beginning of the pandemic. CCP has started the unrestricted warfare against the US in variable sneak ways like Information Warfare, Cyber-Attack, Propaganda Warfare and current Virus Attack. CCP has initiated PR campaign to cover up after COVID-19 outbreak. Since CCP has unleashed the virus to the world they do not want it to be stopped soon at such a low cost. China's economy power has facilitated CCP to have full economic strength to control WHO and some renowned scientists to speak up for CCP. In China HCQ can pose a huge impact on the traditional Chinese medicine market with hundreds of millions consumers. In the US because HCQ was recommended by President Trump, those who oppose him defame HCQ just for the sake of opposing him. For pharmaceutical companies like AbbVie and Gilead this low cost effective medicine can be a devastating blow to their sales. The interest groups behind these companies will take any measures to prevent from its use. Therefore WHO removed HCQ in the trial medicine list. The Lancet posted an article to defame HCQ. FDA and NIH keep the warning of the HCQ's side effect. University of Oxford released news to defame HCQ.
at the beginning of the pandemic. CCP has started the unrestricted warfare against the US in variable sneak ways like Information Warfare, Cyber-Attack, Propaganda Warfare and current Virus Attack. CCP has initiated PR campaign to cover up after COVID-19 outbreak. Since CCP has unleashed the virus to the world they do not want it to be stopped soon at such a low cost. China's economy power has facilitated CCP to have full economic strength to control WHO and some renowned scientists to speak up for CCP. In China HCQ can pose a huge impact on the traditional Chinese medicine market with hundreds of millions consumers. In the US because HCQ was recommended by President Trump, those who oppose him defame HCQ just for the sake of opposing him. For pharmaceutical companies like AbbVie and Gilead this low cost effective medicine can be a devastating blow to their sales. The interest groups behind these companies will take any measures to prevent from its use. Therefore WHO removed HCQ in the trial medicine list. The Lancet posted an article to defame HCQ. FDA and NIH keep the warning of the HCQ's side effect. University of Oxford released news to defame HCQ.|
According to drugs.com: At least 50 kg: 800 mg salt (620 mg base) orally on day 1, followed by 400 mg salt (310 mg base) orally once a day. Total duration of therapy: 4 to 7 days, based on clinical evaluation.
According to Johns Hopkins University treatment (children and adolescents): 6.5 mg/kg q12h on day 1 (maximum initial dose = 400 mg q12h), followed by 3.25 mg/kg q12h on days 2 - 5 (maximum dose = 200 mg q12h). If tolerated, consider condensing maintenance dose to once daily in hospitalized patients (ex. 6.5 mg/kg q24h instead of 3.25 mg/kg.q12h) The average wholesale price: 1.88-12.54 USD.
Side effect: Headache Gastrointestinal complaints (diarrhea, anorexia, nausea, abdominal cramps and, on rare occasions, vomiting) Blurring of vision due to a disturbance of accommodation; reversible and dose-dependent, Skin rash, pruritus.
MECHANISM:Chloroquine increases endosomal pH required for virus/cell fusion, as well as interfering with the glycosylation of cellular receptors of SARS-CoV.
In the early in vitro studies, chloroquine was found to block COVID-19 infection at low-micromolar concentration, with a half-maximal effective concentration (EC50) of 1.13 μM and a half-cytotoxic concentration (CC50) greater than 100 μM (4).
The results from more than 100 patients have demonstrated that chloroquine phosphate is superior to the control treatment in inhibiting the exacerbation of pneumonia, improving lung imaging findings, promoting a virusnegative conversion, and shortening the disease course according to the news briefing. Severe adverse reactions to chloroquine phosphate were not noted in the aforementioned patients.
It was an open letter posted on February 29 2020. It has introduced a number of Chinese hospitals that have conducted the trials with inspiring results. The two authors took quick action to collect nationwide trial results and shared these with the rest of the world.
Here are 15 trials registered. 5 of them have cancelled. ChiCTR2000029939 HwaMei Hospital, ChiCTR2000029935 HwaMei Hospital, ChiCTR2000029899 Peking University Third Hospital, ChiCTR2000029898 Peking University Third Hospital, ChiCTR2000029868 Shanghai Ruijin Hospital, ChiCTR2000029803 Renmin Hospital of Wuhan University, ChiCTR2000029740 The First Hospital of Peking University, ChiCTR2000029609 The Fifth Affiliated Hospital of Sun Yat-Sen University, ChiCTR2000029559 Renmin Hospital of Wuhan University, ChiCTR2000029542 Sun Yat sen Memorial Hospital of Sun Yat sen University
Methods: The patients were randomized 1:1 to HCQ group and the control group. Patients in HCQ group were given HCQ 400 mg per day for 5 days plus conventional treatments, while those in the control group were given conventional treatment only. The primary endpoint was negative conversion rate of SARS-CoV-2 nucleic acid in respiratory pharyngeal swab on days 7 after randomization.
Results: One patient in HCQ group developed to severe during the treatment. On day 7, nucleic acid of throat swabs was negative in 13 (86.7%) cases in the HCQ group and 14 (93.3%) cases in the control group (P>0.05). The median duration from hospitalization to virus nucleic acid negative conservation was 4 (1, 9) days in HCQ group, which is comparable to that in the control group [2 (1, 4) days, Z=1.27, P>0.05]. The median time for body temperature normalization in HCQ group was 1 (0, 2) day after hospitalization, which was also comparable to that in the control group [1 (0, 3) day]. Radiological progression was shown on CT images in 5 cases (33.3%) of the HCQ group and 7 cases (46.7%) of the control group, and all patients showed improvement in follow-up examinations.
Conclusions: The prognosis of COVID-19 moderate patients is good. Larger sample size study are needed to investigate the effects of HCQ in the treatment of COVID-19.
Clinical investigation found that high concentration of cytokines were detected in the plasma of critically ill patients infected with SARS-CoV-2, suggesting that cytokine storm was associated with disease severity12. Other than its direct antiviral activity, HCQ is a safe and successful anti-inflammatory agent that has been used extensively in autoimmune diseases and can significantly decrease the production of cytokines and, in particular, pro-inflammatory factors. Therefore, in COVID-19 patients, HCQ may also contribute to attenuating the inflammatory response.
News on WIV's website.
French Confirmed COVID-19
 patients were included in a single arm protocol from early March to March 16th, to receive 600mg of hydroxychloroquine daily and their viral load in nasopharyngeal swabs was tested daily in a hospital setting. patients were included in a single arm protocol from early March to March 16th, to receive 600mg of hydroxychloroquine daily and their viral load in nasopharyngeal swabs was tested daily in a hospital setting.Results:Six patients were asymptomatic, 22 had upper respiratory tract infection symptoms and eight had lower respiratory tract infection symptoms. Twenty cases were treated in this study and showed a significant reduction of the viral carriage at D6-post inclusion compared to controls, and much lower average carrying duration than reported of untreated patients in the literature. Azithromycin added to hydroxychloroquine was significantly more efficient for virus elimination.
Conclusion:Despite its small sample size our survey shows that hydroxychloroquine treatment is significantly associated with viral load reduction/disappearance in COVID-19 patients and its effect is reinforced by azithromycin.
The trial was led by Didier Raoult, 68 years old French physician, microbiologist and professor in infectious disease of Aix-Marseille University. He has been the chairman of Research Unit on Emerging Infectious and Tropical Diseases. He got significant global attention for promoting hydroxychloroquine as treatment for the COVID-19.
Experimental: Chloroquine or Hydroxychloroquine: In Asia, the participant will receive chloroquine. In Europe and Africa, the participant will receive hydroxychloroquine.
Drug:Chloroquine or Hydroxychloroquine.
A loading dose of 10 mg base/ kg followed by 155 mg daily (250mg chloroquine phosphate salt or 200mg of or hydroxychloroquine sulphate) will be taken for 3 months.
Estimated Enrollment: 40000 participants,
Actual Study Start Date: April 29, 2020. Estimated Primary Completion Date:April 2021. The dose in RECOVERY is Hydroxychloroquine (155mg base per 200mg tablet): Initial dose: 4 tablets 6 hours later: 4 tablets, 12 hours: 2 tablets, 24 hours: 2 tablets Thereafter: 2 tablets every 12 hours for a total of 10 days, The first day loading dose in RECOVERY (1.86g base) is twice the first day loading dose for treating malaria. This dose has been selected based on the available data of the IC50 for SARS-CoV-2.
The first day loading dose in RECOVERY (1.86g base) is twice the first day loading dose for treating malaria. This dose has been selected based on the available data of the IC50 for SARS-CoV-2. The objective is to reach plasma concentrations that are inhibitory to the virus as soon as safely possible.
RecoveryTrial is the world largest clinical trial in more than 11500 patients in the UK in name of University of Oxford. It is led by Peter Horby. He insisted in promoting lopinavir/ritonavir rather than remdesivir in the trial.
Hydroxychloroquine can help patients efficiently suppressing immune system in order to prevent from occurrence of cytokine storms caused by virus infection. The does is much higher than the dose in the guidance. It is hard believe there is such a crucially large inconsistency in the protocol and the intervention data sheet and both of them were made by University of Oxford.
There is only the intervention sheet (This file has been deleted.) on recoverytrial.net. No any other online trial document made by University of Oxford can be found.
On April 2 Henry Ford Health System decided to lead the first large-scale study named WHIP COVID-19 Study with expected 3000+ in the United States of the effectiveness of an anti-malarial drug in preventing COVID-19 in healthcare workers and first responders who volunteer to participate. The trial was registered on April 10.
"Participants will be provided with weekly dosing of hydroxychloroquine (HCQ) 400mg po q weekly, daily dosing of HCQ 200mg po q daily following a loading dose of 400mg day 1, or placebo. Participants will receive monitoring at each study week visit to assess for the development of COVID-19 related symptoms, COVID-19 clinical disease, and medication side effects".
On July 2 the trial result was covered here with "The study analyzed 2,541 patients hospitalized among the system’s six hospitals between March 10 and May 2 and found 13% of those treated with hydroxychloroquine died while 26% of those who did not receive the drug died."
"Among all the patients in the study, there was an overall in-hospital mortality rate of 18%, and many who died had underlying conditions, the hospital system said. Globally, the mortality rate for hospitalized patients is between 10% and 30%, and 58% among those in the ICU or on a ventilator."
On April 2 Chicago Tribune has covered Dr. Vladimir Zelenko, 46, a mild-mannered family doctor with offices near the village. Since early March, his clinics had treated people with coronaviruslike symptoms, and he had developed an experimental treatment consisting of an antimalarial medication called hydroxychloroquine, the antibiotic azithromycin and zinc sulfate.
After testing this three-drug cocktail on hundreds of patients, some of whom had only mild or moderate symptoms when they arrived, Zelenko claimed that 100% of them had survived the virus with no hospitalizations and no need for a ventilator.
It has been covered by medicine.com as well.
This physician has not published any article on this trial. He developed this experimental three-drug cocktail treatment inspired by the success of trial in Korea. His treatment proved its clinical efficacy
Studies on prophylaxis of SARS-CoV-2 infection
|
- For the patients with long-term chronic cardiovascular and cerebrovascular diseases COVID-19 infection will cause complication and be deadly for them.
- The studies has found out the COVID-19 has mutated into two major sub types S and L. People got infected by sub-type S shows a mild symptom and can be self-recovered without any medication. Most of the infected patients just need to quarantine and wait for recovery. However the sub-type L virus is more aggressive and can lead to mortality. The vast majority of them die of respiratory failure.
- Researchers of MIT and Stanford University archived with this finding "SARS-CoV-2 could exploit species-specific interferon-driven upregulation of ACE2, a tissue-protective mediator during lung injury, to enhance infection".
- Another finding shows blood appeared to be abnormally sticky in COVID-19's patient. Research has confirmed people get infected by COVID-19 can cause coagulopathy and antiphospholipid occurred. Antiphospholipid Antibodies's occurrence can form clot and lead to sudden death.
 University of Oxford. Kome Gbinigie, Kerstin Frie, the two Wellcome Trust funded DPhil students of Nuffield Department of Medicine of University of Oxford published the first article《Chloroquine and hydroxychloroquine: Current evidence for their effectiveness in treating COVID-19》 on March 25 and the second one《Should chloroquine and hydroxychloroquine be used to treat COVID-19? A rapid review》on April 8. They did not have any practical experience in HCQ. They did not engage in any trial. They searched relevant articles online, cited the conclusions and data they need and then made their own conclusion. Both of the articles have not been reviewed by peers before the publication. The first one fairly presented the global trial results. Within one month however the two authors changed their mind and wrote the second article to defame HCQ. The second article was funded by Wellcome Trust. On the same day the two authors posted news on Nuffield Department of Medicine website. All the authors referred in the second article are from two Chinese universities, one French university and they have much advanced academic qualifications and more practical experience than the two DPhil students'. On April 14 the same two DPhil students published the third article to promote lopinavir/ritonavir as an effective solution for CONVID-19 treatment. During the global effort fighting against COVID-19 funding won over science to be more decisive.
University of Oxford. Kome Gbinigie, Kerstin Frie, the two Wellcome Trust funded DPhil students of Nuffield Department of Medicine of University of Oxford published the first article《Chloroquine and hydroxychloroquine: Current evidence for their effectiveness in treating COVID-19》 on March 25 and the second one《Should chloroquine and hydroxychloroquine be used to treat COVID-19? A rapid review》on April 8. They did not have any practical experience in HCQ. They did not engage in any trial. They searched relevant articles online, cited the conclusions and data they need and then made their own conclusion. Both of the articles have not been reviewed by peers before the publication. The first one fairly presented the global trial results. Within one month however the two authors changed their mind and wrote the second article to defame HCQ. The second article was funded by Wellcome Trust. On the same day the two authors posted news on Nuffield Department of Medicine website. All the authors referred in the second article are from two Chinese universities, one French university and they have much advanced academic qualifications and more practical experience than the two DPhil students'. On April 14 the same two DPhil students published the third article to promote lopinavir/ritonavir as an effective solution for CONVID-19 treatment. During the global effort fighting against COVID-19 funding won over science to be more decisive. |
 by main stream medias including Bloomberg. It has been not only covered but exaggerated by an article written by a US based Chinese scientist as "The early termination of the hydroxychloroquine arm was based on data from over 4000 patients enrolled and over 1000 deaths observed in the hydroxychloroquine arm and the usual care arm combined. " There are a lot of search results where the media in the world distributed this message on the same day. There is no doubt it was a well-coordinated PR campaign of defaming HCQ. News on Nuffield Department of Medicine website revealed the department received funding from varieties of funding bodies including Li Ka-shing foundation, the world second largest foundation next to Bill Gate Foundation. Li Ka-shing has been offered a lot of profitable business opportunities by CCP therefore he made a great fortune in particular in estate sector in mainland China. Li Ka-shing has donated to build The Li Ka-shing Centre for the department. There has been a deep tie between CCP and Nuffield Department of Medicine.
by main stream medias including Bloomberg. It has been not only covered but exaggerated by an article written by a US based Chinese scientist as "The early termination of the hydroxychloroquine arm was based on data from over 4000 patients enrolled and over 1000 deaths observed in the hydroxychloroquine arm and the usual care arm combined. " There are a lot of search results where the media in the world distributed this message on the same day. There is no doubt it was a well-coordinated PR campaign of defaming HCQ. News on Nuffield Department of Medicine website revealed the department received funding from varieties of funding bodies including Li Ka-shing foundation, the world second largest foundation next to Bill Gate Foundation. Li Ka-shing has been offered a lot of profitable business opportunities by CCP therefore he made a great fortune in particular in estate sector in mainland China. Li Ka-shing has donated to build The Li Ka-shing Centre for the department. There has been a deep tie between CCP and Nuffield Department of Medicine.| Peter Horby is leading trial in the UK |
 for funding on Chinese foreign talent recruiting website. It is owned by state-run Beijing Research Institute of Science and Information. Such a website can be used as an open platform to recruit foreign scholars for stealing intellectual properties for CCP. The two authors Kome Gbinigie and Kerstin Frie have registered their profiles. Martin Landray who is leading the world largest trial together with Peter Horby has registered as well. The profile of the author Mandeep R. Mehra who wrote an article defaming HCQ can be found on this website. The well-known Colombia University virologist W. Ian Lipkin 's profile can be found on this site. George F. Gao and Peter Horby can't be found. That tells the user's profile was created by an individual. It is known CCP has been stealing foreign intellectual properties by recruiting global leading scientists and providing funding to them. CCP has strong enough economic strength and power to buy those scientists to write articles for CCP.
for funding on Chinese foreign talent recruiting website. It is owned by state-run Beijing Research Institute of Science and Information. Such a website can be used as an open platform to recruit foreign scholars for stealing intellectual properties for CCP. The two authors Kome Gbinigie and Kerstin Frie have registered their profiles. Martin Landray who is leading the world largest trial together with Peter Horby has registered as well. The profile of the author Mandeep R. Mehra who wrote an article defaming HCQ can be found on this website. The well-known Colombia University virologist W. Ian Lipkin 's profile can be found on this site. George F. Gao and Peter Horby can't be found. That tells the user's profile was created by an individual. It is known CCP has been stealing foreign intellectual properties by recruiting global leading scientists and providing funding to them. CCP has strong enough economic strength and power to buy those scientists to write articles for CCP. leading the clinical trial in the UK. The death toll in the graphics shown in below does not appear to be improved significantly. Scientific research has been interfered by the funding bodies. The research results have been distorted and turned to be misleading. It is a tragedy not only for the UK but the world. Scientists with evil mind are threats to the world.
leading the clinical trial in the UK. The death toll in the graphics shown in below does not appear to be improved significantly. Scientific research has been interfered by the funding bodies. The research results have been distorted and turned to be misleading. It is a tragedy not only for the UK but the world. Scientists with evil mind are threats to the world. 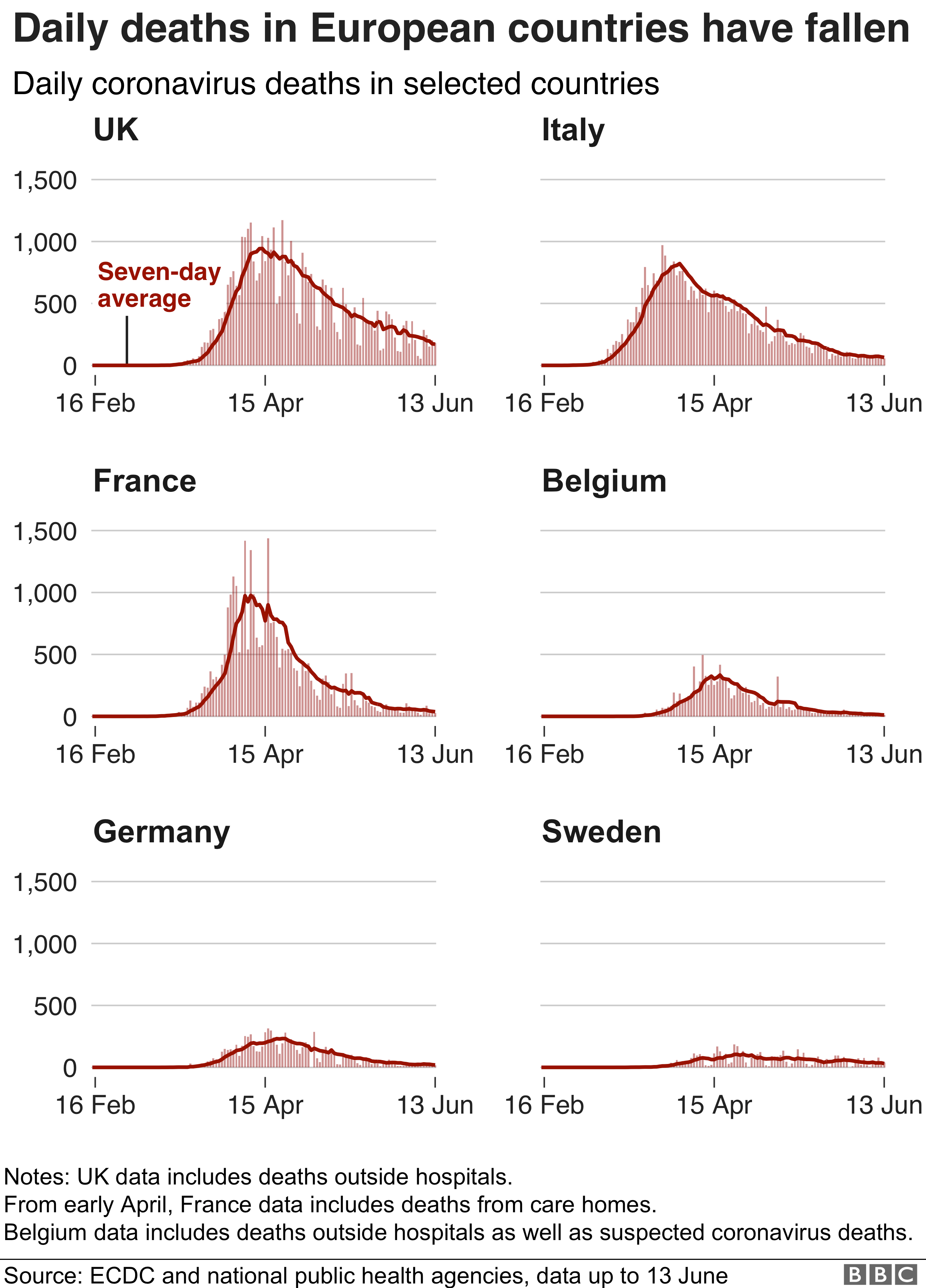 |




