The Seyferth–Gilbert homologation is a chemical reaction of an aryl ketone 1 (or aldehyde) with dimethyl (diazomethyl)phosphonate 2 and potassium tert-butoxide to give substituted alkynes 3.[1][2] Dimethyl (diazomethyl)phosphonate 2 is often called the Seyferth–Gilbert reagent.[3]

This reaction is called a homologation because the product has exactly one additional carbon more than the starting material.
Deprotonation of the Seyferth–Gilbert reagent A gives an anion B, which reacts with the ketone to form the oxaphosphetane D. Elimination of dimethylphosphate E gives the vinyl diazo-intermediate Fa and Fb. The generation of nitrogen gas gives a vinyl carbene G, which via a 1,2-migration forms the desired alkyne H.

The dimethyl (diazomethyl)phosphonate carbanion can be generated in situ from dimethyl-1-diazo-2-oxopropylphosphonate (also called the Ohira-Bestmann reagent) by reaction with methanol and potassium carbonate as the base by cleavage of the acetyl group as methyl acetate. Reaction of Bestmann's reagent with aldehydes gives terminal alkynes often in very high yield and fewer steps than the Corey–Fuchs reaction.[4][5]

The use of the milder potassium carbonate makes this procedure much more compatible with a wide variety of functional groups.
Improved in situ generation of the Ohira-Bestmann reagent
Recently a safer and more scalable approach has been developed for the synthesis of alkynes from aldehydes. This protocol takes advantage of a stable sulfonyl azide, rather than tosyl azide, for the in situ generation of the Ohira−Bestmann reagent.[6]
Another modification for less reactive aldehydes is made by replacement of potassium carbonate with caesium carbonate in MeOH and results in a drastic[quantify] yield increase.[7]
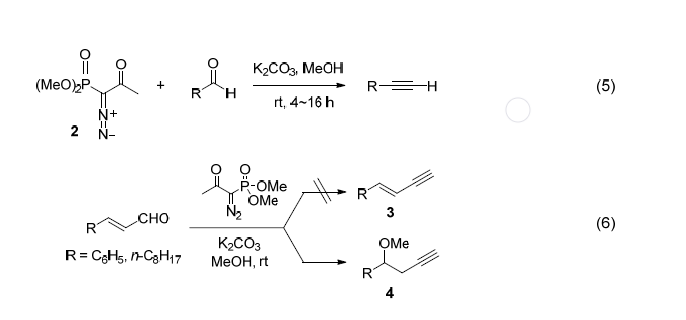
Seyferth-Gilbert反应被Vandewalle小组用于维生素D3衍生物的合成(式 3)。在埃坡霉素B和D及其类似物的合成中,该方法也被用于构筑重要片段,收率达 80% (式 4)。

Ohira在1989年报道了一种改进方法。该法以1-重氮-2-氧代-丙基磷酸二甲酯(2)为试剂,在氩气氛下现场去除酰基生成Seyferth试剂(1)的碳负离子。随后,Bestmann系统地研究了这一“一瓶”方法,发现该法只需简单地通过室温下把磷酸酯加入K2CO3与醛的甲醇溶液就可合成炔,从而避免使用强碱、低温和惰性气体等条件。在这一条件下,脂肪醛和芳醛均可顺利实现增碳,收率为72%~97%(式 5)。与α,β-不饱和醛反应虽然也可实现增碳,但产物为甲氧基共轭加成产物4(式 6)。