酯在碱性条件下水解为羧酸在有机合成化学中非常常见(主要为甲酯或乙酯,而叔丁酯则通常采用酸性水解,苄基酯采用氢化条件),常用的碱性条件是LiOH/THF/MeOH/H2O。但当底物中含有对碱敏感的基团(如氰基)时,使用LiOH水解酯的过程中则容易引入杂质(氰基水解等),那么该如何解决呢?
方法一:TMSOK体系
TMSOK体系也是一个温和水解酯基的条件,下图原料1在NaOH条件下水解甲酯时,会生成氰基也被水解成甲酰胺的副产物。
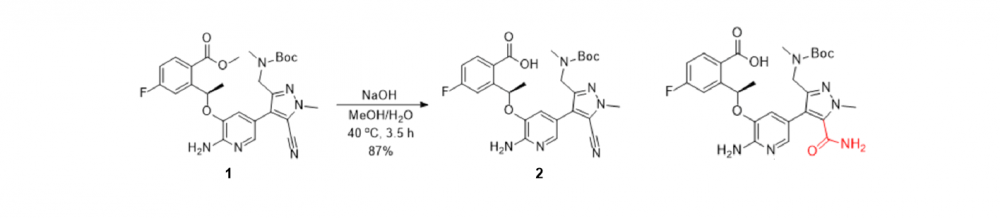
鉴于氰基的因素,后期采用TMSOK进行水解,反应的选择性就非常好,在选择性水解甲酯的同时很好地保护氰基,仅用乙腈作溶剂,水解后的产物直接以钾盐晶体的形式从反应液中析出,经测定,该钾盐晶体的纯度超过了98%。(Org. Process Res. Dev. 2018, 22, 9, 1289-1293.)
2005年,Scripps研究所K. C. Nicolaou(KCN大牛)小组在ACIE上发表了三甲基氢氧化锡(Me3SnOH)水解甲酯的方法,Me3SnOH作为一种温和的碱,反应选择性非常不错,底物中的手性中心能够得到最大程度的保留,并且可以很好地保护对强碱敏感的基团不被破坏;当底物中同时存在多种类型的酯基(甲酯、乙酯、异丙酯等)时,Me3SnOH还可实现选择性水解甲酯(下图 entry 9-12)。(Angew. Chem. Int. Ed. 2005, 44, 1378 –1382)
在水解氨基酸衍生物方面,与其它水解甲酯的方法相比,Me3SnOH的条件能够最大程度上保留手性中心。即使在上述介绍的方法一(TMSOK体系)下,产物的dr值也有颇大的变化。
方法三:LiBr/Et3N/H2O-CH3CN体系
该体系本质上与LiOH水解酯基一样,只不过采用LiBr/Et3N/H2O-CH3CN体系是在原位生成低浓度的LiOH,从而提高水解酯基的选择性,底物中的酰胺键也能得到保留。(Tetrahedron Letters. 2007, 48, 2497–2499)
实验操作:the ester (typically 0.5–1 g) was dissolved in CH3CN (10 ml/g of ester) containing 2 vol % of water. Triethylamine (3 equiv) was added followed by the addition of LiBr (10 equiv). The mixture was stirred vigorously at room temperature, except in one case, in which the reaction was performed at reflux (compound 3!4). When full conversion was achieved, as judged by LC analysis of the crude mixtures, the acids (or alcohol 10) were isolated after a simple work-up procedure. Alternatively, the acids were isolated after purification by preparative HPLC.
以上部分内容源于公众号Synthetic Chemistry,若有侵权请告知。




