Selectfluor, a trademark of Air Products and Chemicals, is a reagent in chemistry that is used as a fluorine donor. This compound is a derivative of the nucleophillic base DABCO. It is a colourless salt that tolerates air and even water. It has been commercialized for use for electrophilic fluorination.
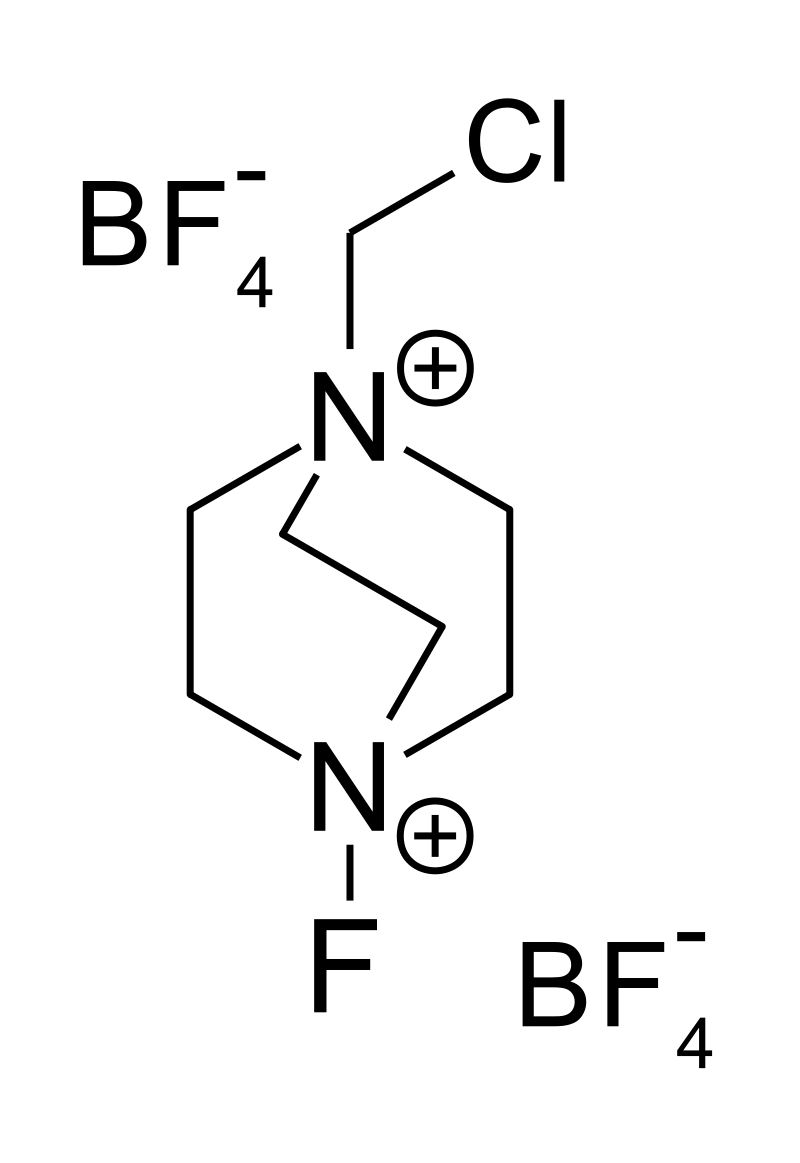
Selectfluor is synthesized by the N-alkylation of diazabicyclo[2.2.2]octane (DABCO) with dichloromethane, followed by ion exchange with sodium tetrafluoroborate (replacing the chloride counterion for the tetrafluoroborate). The resulting salt is treated with elemental fluorine and sodium tetrafluoroborate:[2]
The cation is often depicted with one skewed ethylene ((CH2)2) group. In fact, these pairs of CH2 groups are eclipsed so that the cation has idealized C3h symmetry.




