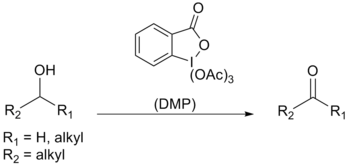
Dess–Martin periodinane (DMP) is a chemical reagent used in the Dess–Martin oxidation, oxidizing primary alcohols to aldehydes and secondary alcohols to ketones. This periodinane has several advantages over chromium- and DMSO-based oxidants that include milder conditions (room temperature, neutral pH), shorter reaction times, higher yields, simplified workups, high chemoselectivity, tolerance of sensitive functional groups, and a long shelf life. However, use on an industrial scale is made difficult by its cost and its potentially explosive nature. It is named after the American chemists Daniel Benjamin Dess and James Cullen Martin who developed the reagent in 1983. It is based on IBX, but due to the acetate groups attached to the central iodine atom, DMP is much more reactive than IBX and is much more soluble in organic solvents.

James Cullen Martin (January 14, 1928 – April 20, 1999) was an American chemist. Known in the field as "J.C.", he specialized in physical organic chemistry with an emphasis on main group element chemistry.
Martin received his undergraduate and master's degree at Vanderbilt University. His PhD work was conducted with Paul Bartlett at Harvard. Most of his professional career was at the University of Illinois at Urbana-Champaign, where he was a colleague of Roger Adams, Speed Marvel, David Y. Curtin, Nelson J. Leonard, and Reynold C. Fuson. Late in his career, he moved back to Vanderbilt, but soon succumbed to poor health.
Professor Martin is best known for his work on bonding of main group elements. He is responsible for the hexafluorocumyl alcohol derived "Martin" bidentate ligand and a tridentate analog. With his doctoral student Daniel Benjamin Dess, he invented the Dess–Martin periodinane that is used for selective oxidation of alcohols. He is also known for the creation of the Martin's sulfurane. His later work included studies of the hexaiodobenzene dication that indicated σ-delocalization ("aromaticity") between the iodine atoms.





