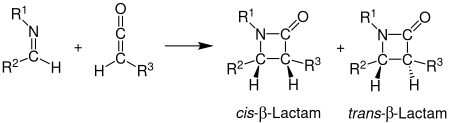
The Staudinger synthesis, also called the Staudinger ketene-imine cycloaddition, is a chemical synthesis in which an imine 1 reacts with a ketene 2 through a non-photochemical 2+2 cycloaddition to produce a β-lactam 3. The reaction carries particular importance in the synthesis of β-lactam antibiotics. The Staudinger synthesis should not be confused with the Staudinger reaction, a phosphine or phosphite reaction used to reduce azides to amines.

The first step is a nucleophilic attack by the imine nitrogen on the carbonyl carbon to generate a zwitterionic intermediate. Electron-donating groups on the imine facilitate this step, while electron-withdrawing groups impede the attack. The second step is either an intramolecular nucleophilic ring closure or a conrotatory electrocyclic ring closure. The second step is different from typical electrocyclic ring closures as predicted by the Woodward–Hoffmann rules. Under photochemical and microwave conditions the intermediate's 4π-electron system cannot undergo a disrotatory ring closure to form the β-lactam, possibly because the two double bonds are not coplanar. Some products of the Staudinger synthesis differ from those predicted by the torquoelectronic model. In addition, the electronic structure of the transition state differs from that of other conrotary ring closures. There is evidence from computational studies on model systems that in the gas phase the mechanism is concerted.

The stereochemistry of the Staudinger synthesis can be difficult to predict because either step can be rate-determining. If the ring closure step is rate-determining, stereochemical predictions based on torquoselectivity are reliable. Other factors that affect the stereochemistry include the initial regiochemistry of the imine. Generally, (E)-imines form cis β-lactams while (Z)-imines form trans β-lactams. Other substituents affect the stereochemistry as well. Ketenes with strong electron-donating substituents mainly produce cis β-lactams, while ketenes with strong electron-withdrawing substituents generally produce trans β-lactams. The ketene substituent affects the transition state by either speeding up or slowing down the progress towards the β-lactam. A slower reaction allows for the isomerization of the imine, which generally results in a trans product.