Schwartz's reagent

Schwartz's reagent is the common name for the organozirconium compound with the formula (C5H5)2ZrHCl, sometimes called zirconocene hydrochloride or zirconocene chloride hydride, and is named after Jeffrey Schwartz, a chemistry professor at Princeton University.This metallocene is used in organic synthesis for various transformations of alkenes and alkynes。
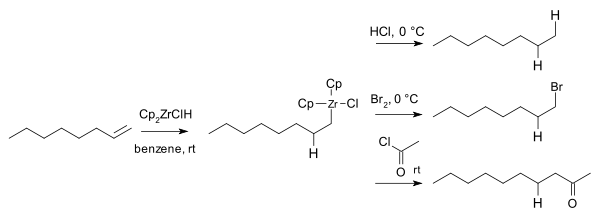
In 1974 Hart and Schwartz reported that the organozirconium intermediates react with electrophiles such as hydrochloric acid, bromine and acid chlorides to give the corresponding alkane, bromoalkanes, and ketones:
Zhao and Snieckus had deeloped an advantageous in situ protocol (Cp2ZrCl2/LiAlH(OBu-t)3 for the generation of the Schwartz reagent which provides a general, convenient, and economic method for the reduction of aliphatic, aromatic, and heteroaromatic tertiary amides to the corresponding aldehydes and the regioselective hydrozirconation−iodination of alkynes and alkenes:
Stryker's reagent
Stryker's reagent ([(PPh3)CuH]6), also known as the Osborn complex, is a hexameric copper hydride ligated with triphenylphosphine. It is a brick red, air-sensitive solid. Stryker's reagent is a mildly hydridic reagent, used in homogeneous catalysis of conjugate reduction reactions of enones, enoates, and related substrates.

A conjugate reduction with Strykers reagent to form copper enolates, followed by an intramolecular aldol reaction, was used in the efficient one-pot generation of five- and six-membered carbocycles. This methodology has provided beta-hydroxyketones diastereoselectively, and in good yields, at low temperatures and without the need for a dehydration step.[ Chiu, P.; Szeto, C. P.; Geng, Z.; Cheng, K. F. Tetrahedron Lett. 2001, 42, 4091]
Stryker's reagent allows for the stoichiometric conjugate reduction of a,b-unsaturated ketones or lactones without affecting isolated alkenes, carbonyl groups, halogens or typical oxygenated functionalities under the reaction conditions.[(1) Mahoney, W. S.; Brestensky, D. M.; Stryker, J. M. J. Am. Chem. Soc. 1988, 110, 291. (2) Koenig, T. M.; Daeuble, J. F.; Brestensky, D. M.; Stryker, J. M. Tetrahedron Lett. 1990, 31, 3237. (3) Brestensky, D. M.; Stryker, J. M. Tetrahedron Lett. 1989, 30, 5677. (4) Ziegler, F. E.; Tung, J. S. J. Org. Chem. 1991, 56, 6530. (5) Musicki, B.; Widlanski, T. S. Tetrahedron Lett. 1991, 32, 1267. (6) Meyers, A. I.; Elworthy, T. R. J. Org. Chem. 1992, 57, 4732. (7) Aicher, T. D.; Buszek, K. R.; Fang, F. G.; Forsyth, C. J.; Jung, S. H.; Kishi, Y.; Matelich, M. C.; Scola, P. M.; Spero, D. M.; Yoon, S. K. J. Am. Chem. Soc. 1992, 57, 6693. (8) Tao, C.; Donaldson, W. A. J. Org. Chem. 1993, 58, 2134]
Selective reduction of alkynes to the corresponding alkenes can also be achieved with Stryker's reagent. Terminal alkynes are reduced at room temperature, while unactivated internal alkynes react only at elevated temperatures. Protection of propargylic alcohol functionality is usually unnecessary, although in sterically hindered cases, fragmentation is sometimes competitive[Daeuble, J. F.; McGettigan, C.; Stryker, J. M. Tetrahedron Lett. 1990, 31, 2397]
Tributyltin Hydride
Organotin hydrides are very good radical reducing agents due to the relatively weak, nonionic bond between tin and hydrogen (Bu3SnH 74 kcal/mol) that can cleave homolytically.

However, these compounds are plagued by their high toxicity and high fat solubility (lipophilicity). Therefore, with few exceptions, the use of tin hydrides should be avoided. The catalytic use of this reagents with a suitable second reducing agent, or the use of radical H-donors such as indium hydrides and silanes [especially tris(trimethylsilyl)silane] are possible alternatives.
Tin is characterized by a pronounced affinity for sulfur, which can be exploited in various reactions:







