Immunology and virology
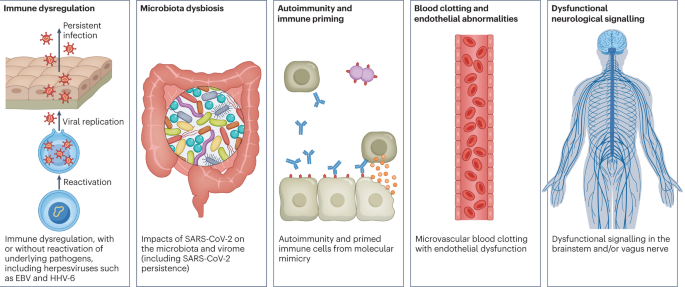
Studies looking at immune dysregulation in individuals with long COVID who had mild acute COVID-19 have found T cell alterations, including exhausted T cells18, reduced CD4+ and CD8+ effector memory cell numbers18,19 and elevated PD1 expression on central memory cells, persisting for at least 13 months19. Studies have also reported highly activated innate immune cells, a lack of naive T and B cells and elevated expression of type I and type III interferons (interferon-β (IFNβ) and IFNλ1), persisting for at least 8 months20. A comprehensive study comparing patients with long COVID with uninfected individuals and infected individuals without long COVID found increases in the numbers of non-classical monocytes, activated B cells, double-negative B cells, and IL-4- and IL-6-secreting CD4+ T cells and decreases in the numbers of conventional dendritic cells and exhausted T cells and low cortisol levels in individuals with long COVID at a median of 14 months after infection18. The expansion of cytotoxic T cells has been found to be associated with the gastrointestinal presentation of long COVID27. Additional studies have found elevated levels of cytokines, particularly IL-1β, IL-6, TNF and IP10 (refs. 40,41), and a recent preprint has reported persistent elevation of the level of CCL11, which is associated with cognitive dysfunction42. It remains to be seen whether the pattern of cytokines in ME/CFS, where the levels of certain cytokines are elevated in the first 2–3 years of illness but decrease over time without a corresponding decrease in symptoms43, is similar in long COVID.
Multiple studies have found elevated levels of autoantibodies in long COVID27, including autoantibodies to ACE2 (ref. 28) (the receptor for SARS-CoV-2 entry), β2-adrenoceptor, muscarinic M2 receptor, angiotensin II AT1 receptor and the angiotensin 1–7 MAS receptor26. High levels of other autoantibodies have been found in some patients with COVID-19 more generally, including autoantibodies that target the tissue (such as connective tissue, extracellular matrix components, vascular endothelium, coagulation factors and platelets), organ systems (including the lung, central nervous system, skin and gastrointestinal tract), immunomodulatory proteins (cytokines, chemokines, complement components and cell-surface proteins)44. A major comprehensive study, however, did not find autoantibodies to be a major component of long COVID18.
Reactivated viruses, including EBV and HHV-6, have been found in patients with long COVID18,21,22,27 (and have been identified in ME/CFS45), and lead to mitochondrial fragmentation and severely affect energy metabolism46. A recent preprint has reported that EBV reactivation is associated with fatigue and neurocognitive dysfunction in patients with long COVID22.
Several studies have shown low or no SARS-CoV-2 antibody production and other insufficient immune responses in the acute stage of COVID-19 to be predictive of long COVID at 6–7 months, in both hospitalized patients and non-hospitalized patients47,48. These insufficient immune responses include a low baseline level of IgG48, low levels of receptor-binding domain and spike-specific memory B cells, low levels of nucleocapsid IgG49 and low peaks of spike-specific IgG47. In a recent preprint, low or absent CD4+ T cell and CD8+ T cell responses were noted in patients with severe long COVID49, and a separate study found lower levels of CD8+ T cells expressing CD107a and a decline in nucleocapsid-specific interferon-γ-producing CD8+ T cells in patients with long COVID compared with infected controls without long COVID50. High levels of autoantibodies in long COVID have been found to be inversely correlated with protective COVID-19 antibodies, suggesting that patients with high autoantibody levels may be more likely to have breakthrough infections27. SARS-CoV-2 viral rebound in the gut, possibly resulting from viral persistence, has also been associated with lower levels and slower production of receptor-binding domain IgA and IgG antibodies51. There are major differences in antibody creation, seroreversion and antibody titre levels across the sexes, with women being less likely to seroconvert, being more likely to serorevert and having lower antibody levels overall52,53, even affecting antibody waning after vaccination54.
Several reports have pointed towards possible viral persistence as a driver of long COVID symptoms; viral proteins and/or RNA has been found in the reproductive system, cardiovascular system, brain, muscles, eyes, lymph nodes, appendix, breast tissue, hepatic tissue, lung tissue, plasma, stool and urine55,56,57,58,59,60. In one study, circulating SARS-CoV-2 spike antigen was found in 60% of a cohort of 37 patients with long COVID up to 12 months after diagnosis compared with 0% of 26 SARS-CoV-2-infected individuals, likely implying a reservoir of active virus or components of the virus16. Indeed, multiple reports following gastrointestinal biopsies have indicated the presence of virus, suggestive of a persistent reservoir in some patients58,61.
Vascular issues and organ damage
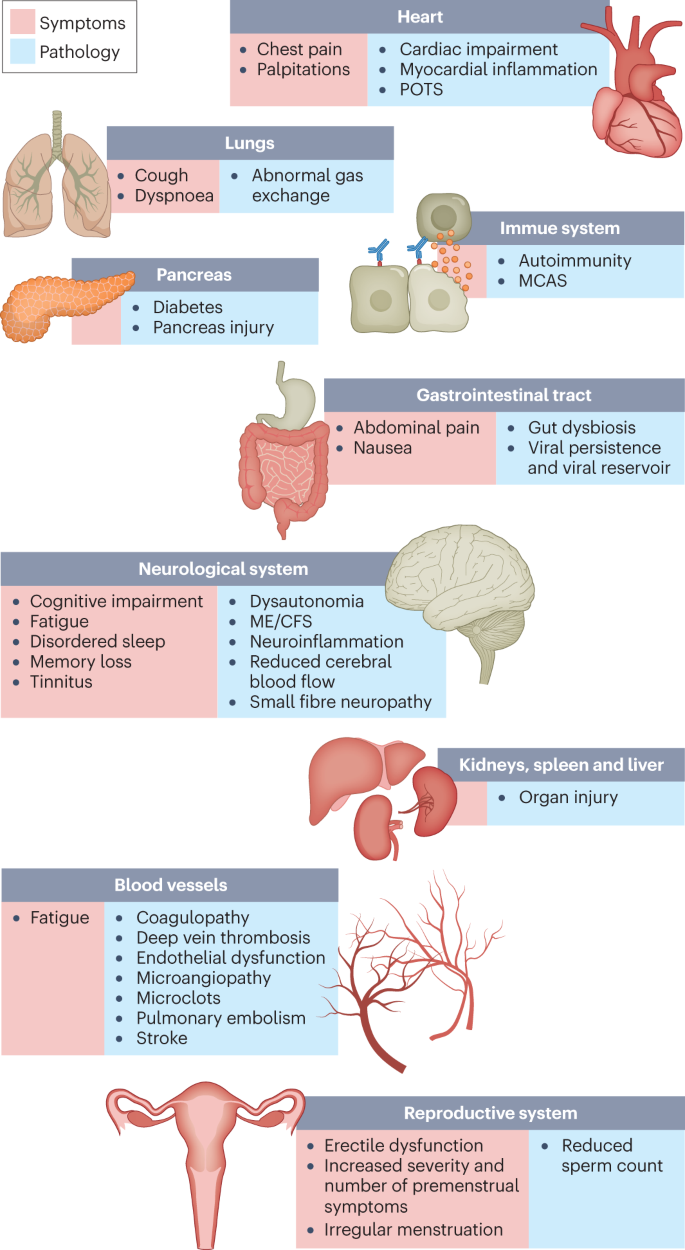
Although COVID-19 was initially recognized as a respiratory illness, SARS-CoV-2 has capability to damage many organ systems. The damage that has been demonstrated across diverse tissues has predominantly been attributed to immune-mediated response and inflammation, rather than direct infection of cells by the virus. Circulatory system disruption includes endothelial dysfunction and subsequent downstream effects, and increased risks of deep vein thrombosis, pulmonary embolism and bleeding events29,30,62. Microclots detected in both acute COVID-19 and long COVID contribute to thrombosis63 and are an attractive diagnostic and therapeutic target. Long-term changes to the size and stiffness of blood cells have also been found in long COVID, with the potential to affect oxygen delivery64. A long-lasting reduction in vascular density, specifically affecting small capillaries, was found in patients with long COVID compared with controls, 18 months after infection65. A study finding elevated levels of vascular transformation blood biomarkers in long COVID also found that the angiogenesis markers ANG1 and P-selectin both had high sensitivity and specificity for predicting long COVID status66.
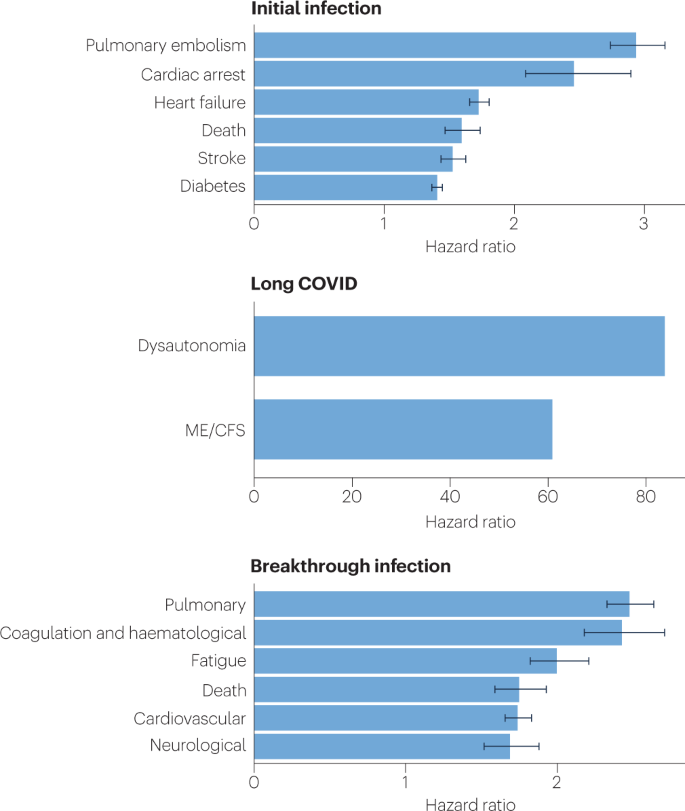
An analysis of the US Department of Veterans Affairs databases (VA data) including more than 150,000 individuals 1 year after SARS-CoV-2 infection indicated a significantly increased risk of a variety of cardiovascular diseases, including heart failure, dysrhythmias and stroke, independent of the severity of initial COVID-19 presentation8 (Fig. 2). Cardiac MRI studies revealed cardiac impairment in 78% of 100 individuals who had a prior COVID-19 episode (investigated an average of 71 days after infection67) and in 58% of participants with long COVID (studied 12 months after infection68), reinforcing the durability of cardiac abnormalities.
Multiple studies have revealed multi-organ damage associated with COVID-19. One prospective study of low-risk individuals, looking at the heart, lungs, liver, kidneys, pancreas and spleen, noted that 70% of 201 patients had damage to at least one organ and 29% had multi-organ damage69. In a 1-year follow-up study, conducted by the same research group with 536 participants, the study authors found that 59% had single-organ damage and 27% multi-organ damage70. A dedicated kidney study of VA data including more than 89,000 individuals who had COVID-19 noted an increased risk of numerous adverse kidney outcomes71. Another VA data analysis, including more than 181,000 individuals who had COVID-19, found that infection also increases the risk of type 2 diabetes9 (Fig. 2). The organ damage experienced by patients with long COVID appears durable, and long-term effects remain unknown.
Neurological and cognitive systems
Neurological and cognitive symptoms are a major feature of long COVID, including sensorimotor symptoms, memory loss, cognitive impairment, paresthesia, dizziness and balance issues, sensitivity to light and noise, loss of (or phantom) smell or taste, and autonomic dysfunction, often impacting activities of daily living7,32. Audiovestibular manifestations of long COVID include tinnitus, hearing loss and vertigo7,72.
In a meta-analysis, fatigue was found in 32% and cognitive impairment was found in 22% of patients with COVID-19 at 12 weeks after infection3. Cognitive impairments in long COVID are debilitating, at the same magnitude as intoxication at the UK drink driving limit or 10 years of cognitive ageing73, and may increase over time, with one study finding occurrence in 16% of patients at 2 months after infection and 26% of patients at 12 months after infection74. Activation of the kynurenine pathway, particularly the presence of the metabolites quinolinic acid, 3-hydroxyanthranilic acid and kynurenine, has been identified in long COVID, and is associated with cognitive impairment74. Cognitive impairment has also been found in individuals who recovered from COVID-19 (ref. 75), and at higher rates when objective versus subjective measures were used3, suggesting that a subset of those with cognitive impairment may not recognize and/or report their impairment. Cognitive impairment is a feature that manifests itself independently of mental health conditions such as anxiety and depression74,76, and occurs at similar rates in hospitalized and non-hospitalized patients74,76. A report of more than 1.3 million people who had COVID-19 showed mental health conditions such as anxiety and depression returned to normal over time, but increased risks of cognitive impairment (brain fog), seizures, dementia, psychosis and other neurocognitive conditions persisted for at least 2 years77.
Possible mechanisms for these neuropathologies include neuroinflammation, damage to blood vessels by coagulopathy and endothelial dysfunction, and injury to neurons32. Studies have found Alzheimer disease-like signalling in patients with long COVID78, peptides that self-assemble into amyloid clumps which are toxic to neurons79, widespread neuroinflammation80, brain and brainstem hypometabolism correlated with specific symptoms81,82 and abnormal cerebrospinal fluid findings in non-hospitalized individuals with long COVID along with an association between younger age and a delayed onset of neurological symptoms83. Multilineage cellular dysregulation and myelin loss were reported in a recent preprint in patients with long COVID who had mild infections, with microglial reactivity similar to that seen in chemotherapy, known as ‘chemo-brain’42. A study from the UK Biobank, including brain imaging in the same patients before and after COVID-19 as well as control individuals, showed a reduction in grey matter thickness in the orbitofrontal cortex and parahippocampal gyrus (markers of tissue damage in areas connected to the primary olfactory cortex), an overall reduction in brain size and greater cognitive decline in patients after COVID-19 compared with controls, even in non-hospitalized patients. Although that study looked at individuals with COVID-19 compared with controls, not specifically long COVID, it may have an implication for the cognitive component of long COVID84. Abnormal levels of mitochondrial proteins as well as SARS-CoV-2 spike and nucleocapsid proteins have been found in the central nervous system85. Tetrahydrobiopterin deficiencies and oxidative stress are found in long COVID as well86.
In the eyes, corneal small nerve fibre loss and increased dendritic cell density have been found in long COVID87,88, as well as significantly altered pupillary light responses89 and impaired retinal microcirculation90. SARS-CoV-2 can infect and replicate in retinal59 and brain91 organoids. Other manifestations of long COVID include retinal haemorrhages, cotton wool spots and retinal vein occlusion92.
Mouse models of mild SARS-CoV-2 infection demonstrated microglial reactivity and elevated levels of CCL11, which is associated with cognitive dysfunction and impaired neurogenesis42. Hamster models exhibited an ongoing inflammatory state, involving T cell and myeloid activation, production of pro-inflammatory cytokines and an interferon response that was correlated with anxiety and depression-like behaviours in the hamsters, with similar transcriptional signatures found in the tissue of humans who had recovered from COVID-19 (ref. 93). Infected non-human primates with mild illness showed neuroinflammation, neuronal injury and apoptosis, brain microhaemorrhages, and chronic hypoxaemia and brain hypoxia94.
Recent reports indicate low blood cortisol levels in patients with long COVID as compared with control individuals, more than 1 year into symptom duration18,27. Low cortisol production by the adrenal gland should be compensated by an increase in adrenocorticotropic hormone (ACTH) production by the pituitary gland, but this was not the case, supporting hypothalamus–pituitary–adrenal axis dysfunction18. This may also reflect an underlying neuroinflammatory process. Low cortisol levels have previously been documented in individuals with ME/CFS.
ME/CFS, dysautonomia and related conditions
ME/CFS is a multisystem neuroimmune illness with onset often following a viral or bacterial infection. Criteria include a “substantial reduction or impairment in the ability to engage in pre-illness levels of occupational, educational, social, or personal activities” for at least 6 months, accompanied by a profound fatigue that is not alleviated by rest, along with postexertional malaise, unrefreshing sleep and cognitive impairment or orthostatic intolerance (or both)95. Up to 75% of people with ME/CFS cannot work full-time and 25% have severe ME/CFS, which often means they are bed-bound, have extreme sensitivity to sensory input and are dependent on others for care96. There is a vast collection of biomedical findings in ME/CFS97,98, although these are not well known to researchers and clinicians in other fields.
Many researchers have commented on the similarity between ME/CFS and long COVID99; around half of individuals with long COVID are estimated to meet the criteria for ME/CFS10,11,29,100, and in studies where the cardinal ME/CFS symptom of postexertional malaise is measured, a majority of individuals with long COVID report experiencing postexertional malaise7,100. A study of orthostatic stress in individuals with long COVID and individuals with ME/CFS found similar haemodynamic, symptomatic and cognitive abnormalities in both groups compared with healthy individuals101. Importantly, it is not surprising that ME/CFS should stem from SARS-CoV-2 infection as 27.1% of SARS-CoV infection survivors in one study met the criteria for ME/CFS diagnosis 4 years after onset102. A wide range of pathogens cause ME/CFS onset, including EBV, Coxiella burnetii (which causes Q fever), Ross River virus and West Nile virus38.
Consistent abnormal findings in ME/CFS include diminished natural killer cell function, T cell exhaustion and other T cell abnormalities, mitochondrial dysfunction, and vascular and endothelial abnormalities, including deformed red blood cells and reduced blood volume. Other abnormalities include exercise intolerance, impaired oxygen consumption and a reduced anaerobic threshold, and abnormal metabolic profiles, including altered usage of fatty acids and amino acids. Altered neurological functions have also been observed, including neuroinflammation, reduced cerebral blood flow, brainstem abnormalities and elevated ventricular lactate level, as well as abnormal eye and vision findings. Reactivated herpesviruses (including EBV, HHV-6, HHV-7 and human cytomegalovirus) are also associated with ME/CFS97,98,103,104.
Many of these findings have been observed in long COVID studies in both adults and children (Box 1). Long COVID research has found mitochondrial dysfunction including loss of mitochondrial membrane potential105 and possible dysfunctional mitochondrial metabolism106, altered fatty acid metabolism and dysfunctional mitochondrion-dependent lipid catabolism consistent with mitochondrial dysfunction in exercise intolerance107, redox imbalance108, and exercise intolerance and impaired oxygen extraction100,109,110. Studies have also found endothelial dysfunction29, cerebral blood flow abnormalities and metabolic changes81,111,112,113 (even in individuals with long COVID whose POTS symptoms abate114), extensive neuroinflammation42,80, reactivated herpesviruses18,21,27, deformed red blood cells64 and many findings discussed elsewhere. Microclots and hyperactivated platelets are found not only in individuals with long COVID but also in individuals with ME/CFS115.
Dysautonomia, particularly POTS, is commonly comorbid with ME/CFS116 and also often has a viral onset117. POTS is associated with G protein-coupled adrenergic receptor and muscarinic acetylcholine receptor autoantibodies, platelet storage pool deficiency, small fibre neuropathy and other neuropathologies118. Both POTS and small fibre neuropathy are commonly found in long COVID111,119, with one study finding POTS in 67% of a cohort with long COVID120.
Mast cell activation syndrome is also commonly comorbid with ME/CFS. The number and severity of mast cell activation syndrome symptoms substantially increased in patients with long COVID compared with pre-COVID and control individuals121, with histamine receptor antagonists resulting in improvements in the majority of patients19.
Other conditions that are commonly comorbid with ME/CFS include connective tissue disorders including Ehlers–Danlos syndrome and hypermobility, neuro-orthopaedic spinal and skull conditions, and endometriosis33,122,123. Evidence is indicating these conditions may be comorbid with long COVID as well. The overlap of postviral conditions with these conditions should be explored further.
Reproductive system
Impacts on the reproductive system are often reported in long COVID, although little research has been done to document the extent of the impact and sex-specific pathophysiology. Menstrual alterations are more likely to occur in women and people who menstruate with long COVID than in women and people who menstruate with no history of COVID and those who had COVID-19 but not long COVID124. Menstruation and the week before menstruation have been identified by patients as triggers for relapses of long COVID symptoms7. Declined ovarian reserve and reproductive endocrine disorder have been observed in people with COVID-19 (ref. 125), and initial theories suggest that SARS-CoV-2 infection affects ovary hormone production and/or the endometrial response due to the abundance of ACE2 receptors on ovarian and endometrial tissue126. Individuals with both COVID-19 and menstrual changes were more likely to experience fatigue, headache, body ache and pain, and shortness of breath than those who did not have menstrual changes, and the most common menstrual changes were irregular menstruation, increased premenstrual symptoms and infrequent menstruation127.
Research on ME/CFS shows associations between ME/CFS and premenstrual dysphoric disorder, polycystic ovarian syndrome, menstrual cycle abnormalities, ovarian cysts, early menopause and endometriosis128,129,130. Pregnancy, postpartum changes, perimenopause and menstrual cycle fluctuations affect ME/CFS and influence metabolic and immune system changes129. Long COVID research should focus on these relationships to better understand the pathophysiology.
Viral persistence in the penile tissue has been documented, as has an increased risk of erectile dysfunction, likely resulting from endothelial dysfunction131. In one study, impairments to sperm count, semen volume, motility, sperm morphology and sperm concentration were reported in individuals with long COVID compared with control individuals, and were correlated with elevated levels of cytokines and the presence of caspase 8, caspase 9 and caspase 3 in seminal fluid132.
Respiratory system
Respiratory conditions are a common phenotype in long COVID, and in one study occurred twice as often in COVID-19 survivors as in the general population2. Shortness of breath and cough are the most common respiratory symptoms, and persisted for at least 7 months in 40% and 20% of patients with long COVID, respectively7. Several imaging studies that included non-hospitalized individuals with long COVID demonstrated pulmonary abnormalities including in air trapping and lung perfusion133,134. An immunological and proteomic study of patients 3–6 months after infection indicated apoptosis and epithelial damage in the airway but not in blood samples135. Further immunological characterization comparing individuals with long COVID with individuals who had recovered from COVID-19 noted a correlation between decreased lung function, systemic inflammation and SARS-CoV-2-specific T cells136.
Gastrointestinal system
Long COVID gastrointestinal symptoms include nausea, abdominal pain, loss of appetite, heartburn and constipation137. The gut microbiota composition is significantly altered in patients with COVID-19 (ref. 23), and gut microbiota dysbiosis is also a key component of ME/CFS138. Higher levels of Ruminococcus gnavus and Bacteroides vulgatus and lower levels of Faecalibacterium prausnitzii have been found in people with long COVID compared with non-COVID-19 controls (from before the pandemic), with gut dysbiosis lasting at least 14 months; low levels of butyrate-producing bacteria are strongly correlated with long COVID at 6 months24. Persisting respiratory and neurological symptoms are each associated with specific gut pathogens24. Additionally, SARS-CoV-2 RNA is present in stool samples of patients with COVID-19 (ref. 139), with one study indicating persistence in the faeces of 12.7% of participants 4 months after diagnosis of COVID-19 and in 3.8% of participants at 7 months after diagnosis61. Most patients with long COVID symptoms and inflammatory bowel disease 7 months after infection had antigen persistence in the gut mucosa140. Higher levels of fungal translocation, from the gut and/or lung epithelium, have been found in the plasma of patients with long COVID compared with those without long COVID or SARS-CoV-2-negative controls, possibly inducing cytokine production141. Transferring gut bacteria from patients with long COVID to healthy mice resulted in lost cognitive functioning and impaired lung defences in the mice, who were partially treated with the commensal probiotic bacterium Bifidobacterium longum25.
Timelines
The onset and time course of symptoms differ across individuals and by symptom type. Neurological symptoms often have a delayed onset of weeks to months: among participants with cognitive symptoms, 43% reported a delayed onset of cognitive symptoms at least 1 month after COVID-19, with the delay associated with younger age83. Several neurocognitive symptoms worsen over time and tend to persist longer, whereas gastrointestinal and respiratory symptoms are more likely to resolve7,74,142. Additionally, pain in joints, bones, ears, neck and back are more common at 1 year than at 2 months, as is paresthesia, hair loss, blurry vision and swelling of the legs, hands and feet143. Parosmia has an average onset of 3 months after the initial infection144; unlike other neurocognitive symptoms, it often decreases over time143.
Few people with long COVID demonstrate full recovery, with one study finding that 85% of patients who had symptoms 2 months after the initial infection reported symptoms 1 year after symptom onset143. Future prognosis is uncertain, although diagnoses of ME/CFS and dysautonomia are generally lifelong.