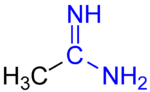
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.
Examples of amidines is DBU:
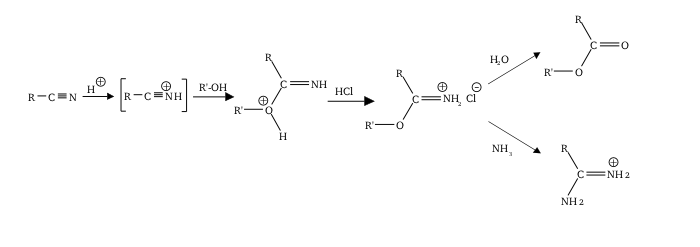
A common route to primary amidines is the Pinner reaction. Reaction of the nitrile with alcohol in the presence of acid gives an iminoether. Treatment of the resulting compound with ammonia then completes the conversion to the amidine.
Amidines are much more basic than amides and are among the strongest uncharged/unionized bases.
Protonation occurs at the sp2 hybridized nitrogen. This occurs because the positive charge can be delocalized onto both nitrogen atoms. The resulting cationic species is known as an amidinium ion and possesses identical C-N bond lengths.





