正文
-
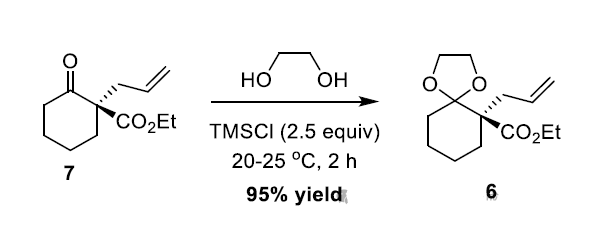
以前的一个案例,目的是保护酮羰基,但是分子底物中有酯基,没有尝试其他保护基源,只研究了乙二醇保护基源,采用经典条件数据不好。 -
乙二醇、催化量对甲苯磺酸、甲苯、梯度升温(因为担心乙二醇和酯交换),随着温度的升高,的确有新点出现,但是一出就两个点,因为不是工艺研究,一个实验就中止了。
看到一篇文献,觉得应该尝试一下,同时分享给大家
-
When azeotropic distillation was applied to remove water in toluene in the presence of catalytic p-toluenesulfonic acid for the formation of the ketal 6, we found that the reaction was difficult to go to full conversion. -
Moreover, the gradual formation of some unknown impurities was observed upon heating with time. -
To overcome this issue, we developed a mild and convenient method for the synthesis of ketals employing commercially available MSTFA [2,2,2-trifluoro-N-methyl-N-(trimethylsilyl)- acetamide] as an effective TMS source. -
The advantage of this new methodology includes the use of stoichiometric diol under mild conditions. However, the application of MSTFA is not economical enough to meet our aim of process sustainability in this case.
评论
目前还没有任何评论
登录后才可评论.




