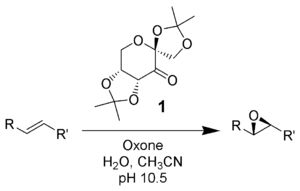
The Shi epoxidation is a chemical reaction described as the asymmetric epoxidation of alkenes with oxone (potassium peroxymonosulfate) and a fructose-derived catalyst (1). This reaction is thought to proceed via a dioxirane intermediate, generated from the catalyst ketone by oxone (potassium peroxymonosulfate). The addition of the sulfate group by the oxone facilitates the formation of the dioxirane by acting as a good leaving group during ring closure. It is notable for its use of a non-metal catalyst and represents an early example of organocatalysis.The reaction was first discovered by Yian Shi (史一安, pinyin: Shǐ Yī-ān) of Colorado State University in 1996.
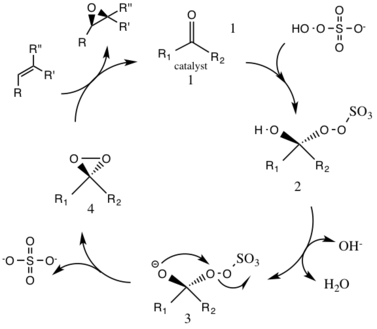
The first step in the catalytic cycle reaction is the nucleophilic addition reaction of the oxone with the ketone group on the catalyst (intermediate 1). This forms the reactive intermediate number 2 species, the Criegee intermediate that can potentially lead to unwanted side reactions, such as the Baeyer-Villiger reaction (see below). The generation of intermediate species number 3 occurs under basic conditions, with a removal of the hydrogen from the hydroxy group to form a nucleophilic oxygen anion. The sulfate group facilitates the subsequent formation of the dioxirane, intermediate species number 4, by acting as a good leaving group during the 3-exo-tet cyclization. The activated dioxirane catalytic species then transfers an oxygen atom to the alkene, leading to a regeneration of the original catalyst.
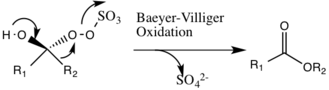
A potential side reaction that may occur is the Baeyer-Villiger reaction of intermediate 2, where there is a rearrangement of the peroxy group that results in the formation of the relative ester. The extent of this side reaction declines with the rise of pH, and increases the nucleophilicity of the oxone, making basic conditions favorable for the overall epoxidation and reactivity of the catalytic species.
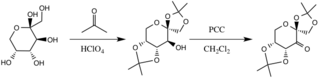
The catalyst is formed by reaction with acetone under basic conditions, with the hydroxyl groups of the fructose ring acting as nucleophiles, their nucleophilicity increased by the basic conditions created by potassium carbonate. The electron withdrawing substituents (alpha-ether groups) encourage the formation of the ketone from the oxidizing agent pyridinium chlorochromate by increasing the electrophilicity of the carbonyl carbon, via a stabilizing delocalization of the forming π C-C bonds into the σ* C-O bonds of the adjacent ethers.
史一安
个人简介
教育经历 1979年9月-1983年7月,南京大学 理学学士学位 1985年9月-1987年6月,加拿大多伦多大学 化学硕士学位 1987年9月-1992年1月,美国斯坦福大学 化学博士学位 工作经历 1992.02-1995.07 美国哈佛大学(博士后) 生物化学 1995.08-2000.06 美国科罗拉多州立大学 助理教授 2000.07-2003.06 美国科罗拉多州立大学 副教授 2003.07-至今 美国科罗拉多州立大学 教授 2008.05-2012.08 中国科学院化学研究所 研究员 2012.09-2017.08 南京大学 教授/博导 2017-至今 常州大学 教授
研究领域
有机合成方法学以及生物活性分子的合成








