Crabtree's catalyst is an organoiridium compound with the formula [C8H12IrP(C6H11)3C5H5N]PF6. It is a homogeneous catalyst for hydrogenation and hydrogen-transfer reactions, developed by Robert H. Crabtree. This air stable orange solid is commercially available and known for its directed hydrogenation to give trans stereoselectivity with respective of directing group.
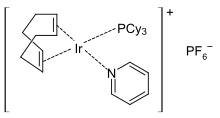
Crabtree’s catalyst is effective for the hydrogenations of mono-, di-, tri-, and tetra-substituted substrates. Whereas Wilkinson’s catalyst and the Schrock–Osborn catalyst do not catalyze the hydrogenation of a tetrasubstituted olefin, Crabtree’s catalyst does so to at high turnover frequencies (table).
-
Turnover frequencies Substrate Wilkinson's catalyst Schrock–Osborn catalyst Crabtree's catalyst Hex-1-ene 650 4000 6400 Cyclohexene 700 10 4500 1-Methylcyclohexene 13 — 3800 2,3-Dimethyl-but-2-ene — — 4000
The catalyst is reactive at room temperature. The reaction is robust without drying solvents or meticulous deoxygenation of the hydrogen. The catalyst is tolerant of weakly basic functional groups such as ester, but not alcohols (see below) or amines. The catalyst is sensitive to proton-bearing impurities.
The catalyst becomes irreversibly deactivated after about ten minutes at room temperature, signaled by appearance of yellow color. One deactivation process involves formation of hydride-bridged dimers. As a consequence, Crabtree's Catalyst is usually used in very low catalyst loading.
Influence of directing functional groups
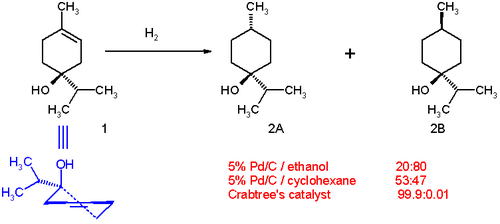
The hydrogenation of a terpen-4-ol demonstrates the ability of compounds with directing groups (the –OH group) to undergo diastereoselective hydrogenation. With palladium on carbon in ethanol the product distribution is 20:80 favoring the cis isomer (2B in Scheme 1). The polar side (with the hydroxyl group) interacts with the solvent. This is due to slight haptophilicity, an effect in which a functional group binds to the surface of a heterogeneous catalyst and directs the reaction. In cyclohexane as solvent, the distribution changes to 53:47 because haptophilicity is no long present (there is no directing group on cyclohexane). The distribution changes completely in favor of the trans isomer 2A when Crabtree's catalyst is used in dichloromethane. This selectivity is both predictable and practically useful. Carbonyl groups are also known to direct the hydrogenation by the Crabtree catalyst to be highly regioselective.


Robert Crabtree




